Among the current sources of water pollution, nitrogen-containing compounds in wastewater are considered one of the most dangerous and difficult-to-control pollutants. High nitrogen levels not only disrupt the ecological balance of aquatic ecosystems but also directly affect human health through the food chain and drinking water sources. Applying measures to remove total nitrogen in wastewater plays an important role in protecting the environment and ensuring safety.
Total nitrogen in wastewater is an indicator representing the overall concentration of nitrogen compounds present in a water sample, including organic nitrogen, ammonium, nitrite, and nitrate. It is one of the key parameters for assessing pollution levels and potential impacts on the natural environment as well as human health. The total nitrogen value is often used as a basis for discharge control and wastewater treatment system design.
In wastewater environments, nitrogen can exist in various forms depending on the source and chemical-biological conditions. The decomposition of organic matter, microbial activity, and chemical reactions in wastewater will transform nitrogen from one form to another. Therefore, determining total nitrogen provides a comprehensive approach to wastewater management rather than focusing on a single nitrogen compound.
What is the basic concept of total nitrogen in wastewater?
Organic nitrogen is found in proteins, amino acids, urea, and other organic compounds from domestic waste and food industry wastewater. When discharged into water, organic nitrogen decomposes slowly under microbial activity. This process can convert organic nitrogen into ammonium, increasing nutrient levels in wastewater.
Ammonium is commonly present in domestic wastewater, particularly from urine, feces, and decomposed organic nitrogen compounds. At low pH, ammonium exists mainly as NH₄⁺, while at high pH, it shifts to NH₃ gas, which is toxic to aquatic organisms. High ammonium concentration not only causes pollution but also indicates strong organic matter decomposition.
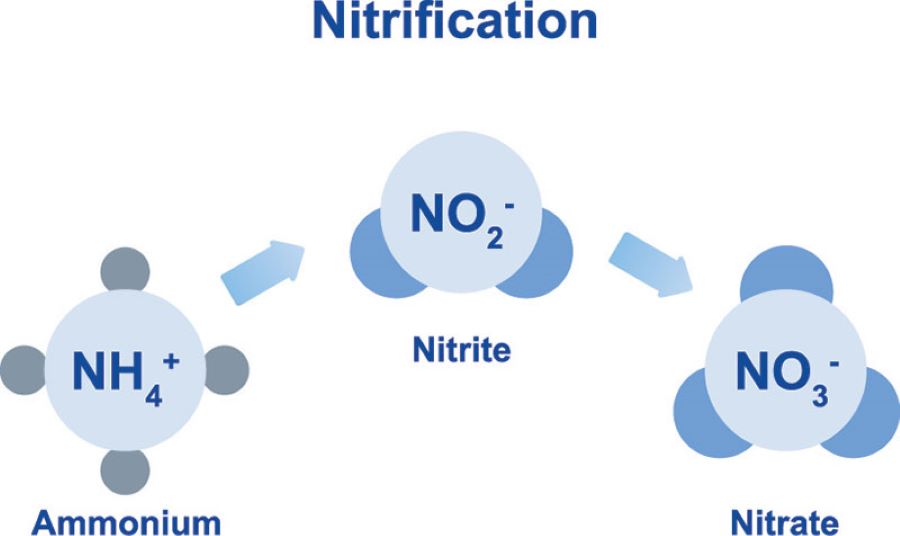
Nitrite is an intermediate product during the oxidation of ammonium to nitrate (nitrification) or the reduction of nitrate back to ammonium (denitrification). Under anaerobic or low-oxygen conditions, nitrite tends to accumulate, becoming toxic to organisms and reducing oxygen transport in human blood (causing methemoglobinemia). Since nitrite is unstable, it usually accounts for a small portion, but its presence indicates incomplete treatment processes.
Nitrate is the final product of nitrogen oxidation in wastewater and is more stable compared to nitrite. Although less toxic than nitrite and ammonium, nitrate acts as a nutrient that promotes eutrophication, leading to harmful algal blooms and reduced water quality. Furthermore, when ingested, nitrate can be converted to nitrite and form nitrosamines, which are potential carcinogens.
Domestic wastewater is one of the main sources of nitrogen, originating from daily activities such as bathing, cooking, washing, and especially human excreta. The main nitrogen components here are urea in urine, protein in feces, and nitrogen-based cleaning agents. Once released into the environment, these compounds quickly decompose into ammonium and other nitrogen forms, increasing the pollution load on urban drainage systems.
In the food processing industry, large amounts of nitrogen come from proteins, amino acids, blood, and organic by-products. The textile and chemical industries use nitrogen-containing compounds in dyes, solvents, and additives, leading to the presence of nitrate and nitrite in wastewater. Particularly, livestock farm wastewater contains high levels of urea, ammonium, and organic nitrogen from animal manure, causing severe pollution if untreated.
Agriculture uses large amounts of inorganic nitrogen fertilizers such as urea, ammonium sulfate, potassium nitrate, and ammonium nitrate. During rainfall or irrigation, nitrogen from fertilizers is washed into rivers, lakes, and canals in the form of nitrate and ammonium. In addition, pesticides and agrochemicals also contain nitrogen compounds, contributing to nitrogen pollution in water sources, especially in intensive farming areas.
Physico-chemical methods are applied when rapid nitrogen removal is needed, especially ammonium. Stripping at high pH removes NH₃ from water through air blowing. Ion exchange uses resins to absorb ammonium ions, which can then be regenerated with salt solutions. Adsorption with activated carbon or zeolite is also effective but tends to be costly and requires material regeneration.
Chemical methods are used when nitrogen concentrations are excessively high and need thorough removal. Ammonium oxidation can be carried out with chlorine, ozone, or other strong oxidants to convert NH₄⁺ into nitrate or nitrogen gas. MAP precipitation involves magnesium and phosphate to form MgNH₄PO₄·6H₂O (struvite), reducing ammonium concentration while recovering fertilizer. Electrochemical processes are also emerging, using electric current to oxidize or reduce nitrogen compounds in wastewater.
Biological treatment is considered a sustainable and cost-effective solution for nitrogen removal. The nitrification process converts ammonium into nitrite and nitrate via Nitrosomonas and Nitrobacter bacteria. Then, denitrification occurs under anoxic conditions, converting nitrate into nitrogen gas (N₂) released into the atmosphere. Particularly, the Anammox (Anaerobic Ammonium Oxidation) process directly removes ammonium and nitrite into nitrogen gas without requiring much carbon source, lowering costs and increasing treatment efficiency.

Most effective methods of treating total nitrogen in wastewater
Total nitrogen in wastewater exists in various forms and originates from multiple sources, ranging from domestic to industrial activities. Excessive nitrogen levels can cause serious consequences for the environment and health, making effective treatment essential. Applying physico-chemical, chemical, and biological methods not only reduces nitrogen pollution but also promotes integrated, sustainable, and eco-friendly wastewater management.